Introduction
Patients with diffuse large B-cell lymphoma (DLBCL) that is refractory to primary immunochemotherapy are well recognized as a high-risk population with poor prognosis, and have a typical median overall survival (OS) of 6-13 months [Farooq U, et al. 2017]. Patients with 'double-hit' (MYCand eitherBCL2orBCL6) and 'triple-hit' (MYC, BCL2andBCL6) genetic aberrations are also considered to be a high-risk/poor prognosis group [Davies A. 2019]. Tafasitamab (MOR208) is an Fc-enhanced, humanized, monoclonal anti-CD19 antibody that is under investigation in combination with lenalidomide (LEN) in autologous stem cell transplant (ASCT)-ineligible patients with relapsed/refractory (R/R) DLBCL in the Phase II L-MIND study (NCT02399085) [Salles G, et al. 2020]. We report efficacy data for patients with high-risk DLBCL (primary refractory disease and double/triple-hit lymphoma [DHL/THL]) who received tafasitamab + LEN in L-MIND (data cut-off: Nov 30, 2019; median follow-up for OS, 31.8 months).
Methods
In the L-MIND study, patients enrolled were aged ≥18 years with R/R DLBCL (1-3 prior systemic therapies, including ≥1 CD20-targeting regimen), with an Eastern Cooperative Oncology Group performance status of 0-2 and ineligible for ASCT.
Patients received 28-day cycles of tafasitamab (12 mg/kg intravenously), once weekly during Cycles 1-3 with a loading dose on Cycle 1 Day 4, then every 2 weeks during Cycles 4-12. LEN (25 mg orally) was administered on Days 1-21 of Cycles 1-12. After Cycle 12, progression-free patients received tafasitamab every 2 weeks until disease progression. The primary endpoint was objective response rate (ORR) (partial response [PR] + complete response [CR]), assessed centrally by an independent review committee. Primary refractory disease was defined as no response (CR or PR) to or progression during or within 6 months of frontline DLBCL therapy.MYC,BCL2andBCL6aberrations were determined via fluorescencein situhybridization using tumor biopsy.
Results
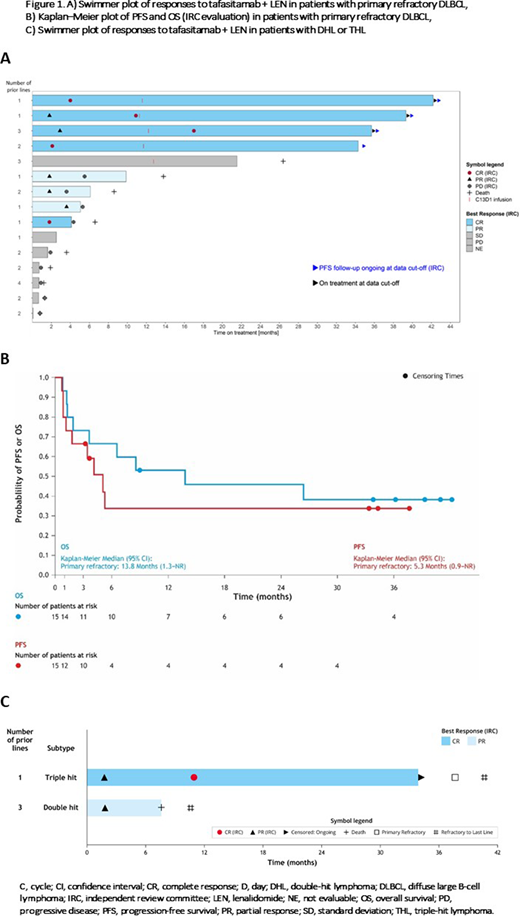
The L-MIND cohort included 15 patients with primary refractory DLBCL and two patients with DHL/THL. Patients with primary refractory DLBCL at baseline had a median age of 73 years (range 48-82; n=9 ≥70 years) and were previously exposed to a median of 2 lines of treatment (range 1-4). Of these patients, ten had stage III/IV disease, ten showed lactate dehydrogenase greater than the upper normal limit, 12 patients had germinal center B-cell DLBCL and eight exhibited intermediate-high or high-risk International Prognostic Index (IPI) status at study baseline. All 15 patients received R-CHOP or equivalent as first-line therapy; two patients previously achieved no response, whereas 13 patients had relapsed within 6 months (ten had achieved a CR and 3 a PR) after frontline therapy.
Six patients had received only 1 prior therapy, whereas nine patients had ≥2 lines before L-MIND enrollment. Median time to progression after first-line therapy was 162 days (range 28-182 days); two of 15 patients had progressed within 90 days. Of the 15 patients, 13 were refractory to their last line of therapy before L-MIND.
In the 15 patients with primary refractory disease, ORR was 53.3% (95% confidence interval [CI]: 26.6-78.7) and the CR rate was 33.3%, with a 30-month duration of response (DOR) rate of 50% (95% CI: 15.2-77.5) - median DOR not reached (NR). Individual response duration is shown in Figure 1 (swimmer plot): four patients who achieved CR remain in remission after >30 months. Median progression-free survival (PFS) was 5.3 months (95% CI: 0.9-NR) and PFS rate at 30 months was 33.9% (95% CI: 11.0-58.8). Median OS was 13.8 months (95% CI: 1.3-NR) and OS at 36 months was 38.1% (95% CI: 14.6-61.6).
Regarding patients with DHL/THL: one with DHL achieved PR only; another patient with THL (also part of the primary refractory subgroup) achieved CR and remains in remission after >30 months.
Conclusions
The combination of tafasitamab + LEN showed encouraging activity, with a clinically meaningful ORR and CR rate in patients with primary refractory DLBCL, and positive responses in DHL and THL. Patients with primary refractory disease were frequently ≥70 years with stage III/IV disease and poor-risk IPI scores. Although these data should be interpreted with caution due to the small patient subgroup sizes, these clinically relevant results warrant further research with this immunotherapy in patients with difficult-to-treat DLBCL.
González-Barca:MorphoSys:Other;Janssen:Consultancy, Honoraria;Sandoz:Consultancy;Gilead:Consultancy;Roche:Honoraria;Takeda:Honoraria;Abbvie:Honoraria;Celgene:Consultancy;Kiowa:Consultancy;Celtrion:Consultancy.Duell:Morphosys:Research Funding.Sancho:Bristol-Myers Squibb:Honoraria;Celgene:Consultancy, Honoraria;Gilead:Consultancy, Honoraria;Janssen:Consultancy, Honoraria;Kern-Pharma:Consultancy, Honoraria;Novartis:Consultancy, Honoraria;Roche:Consultancy, Honoraria;Takeda:Honoraria;Celltrion:Consultancy;Sandoz:Consultancy.Nagy:MorphoSys AG:Patents & Royalties.Abrisqueta:Celgene:Consultancy, Honoraria;AbbVie:Consultancy, Honoraria, Speakers Bureau;Roche:Consultancy, Honoraria, Speakers Bureau;Janssen:Consultancy, Honoraria, Speakers Bureau.Panizo:Clínica Universidad de Navarra:Current Employment;Bristol-Myers Squibb, Kyowa Kirin:Speakers Bureau;Janssen, Roche:Membership on an entity's Board of Directors or advisory committees.Augustin:Morphosys:Research Funding;AstraZeneca:Consultancy, Research Funding;Roche:Consultancy;Novartis:Consultancy, Research Funding;Merck:Consultancy;IPSEN:Consultancy, Research Funding;Pfizer:Consultancy, Research Funding;BMS:Consultancy, Research Funding.Weirather:MorphoSys AG:Current Employment.Ambarkhane:MorphoSys AG:Current Employment.Maddocks:Seattle Genetics:Consultancy, Honoraria;Celgene:Consultancy, Honoraria;Pharmacyclics:Consultancy, Honoraria;Morphosys:Consultancy, Honoraria;ADC Therapeutics, AstraZeneca:Consultancy;BMS:Consultancy, Research Funding;Karyopharm:Consultancy.Kalakonda:Celgene:Research Funding.Salles:BMS/Celgene:Honoraria, Other: consultancy or advisory role;Takeda:Honoraria;Karyopharm:Honoraria;Genmab:Honoraria, Other;Debiopharm:Consultancy, Honoraria, Other: consultancy or advisory role;Autolos:Other: consultancy or advisory role;Abbvie:Other: consultancy or advisory role;Roche:Honoraria, Other: consultancy or advisory role;Novartis:Honoraria, Other: consultancy or advisory role;MorphoSys:Honoraria, Other: consultancy or advisory role;Janssen:Honoraria, Other: consultancy or advisory role;Epizyme:Honoraria, Other: consultancy or advisory role;Kite, a Gilead Company:Honoraria, Other: consultancy or advisory role .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal